

However, you must remember that an enormous amount of energy is required in order for these reactions to occur at all - that is why fusion is not yet a practical source of energy. This is 0.7MeV for fission and 6.2MeV for fusion so it is obvious that fusion is the more effective nuclear reaction. However, the energy per unit mass is more relevant. So it is easy to see that fusion reactions give out more energy per reaction. Considering the mass of the four protons/hydrogen nuclei (4.029106u) and the mass of the Helium produced (4.002603u) we get a mass difference of 0.026503u or 24.69MeV. Finally two Helium-3s fuse forming a Helium nucleus and two hydron nuclei. Then the deuterium fuses with another hydrogen to form Helium-3 and a photon of energy. In a fusion reaction firstly two hydrogens fuse to form a deuterium (an isotope of hydrogen with nucleon no 2), a positron and an electron neutrino. Now looking at the graph the binding energy per nucleon for Uranium is about 7.6MeV and for Barium around 8.3 giving an increase in binding energy during fission of about 0.7MeV per nucleon, or a total of 164.5MeV in total. An example of fission is when a Uranium-235 atom is split by a neutron into a Barium-144 atom, a Krypton-89 atom and three neutrons. However, when comparing the energy released by the fission/fusion of different radioactive isotopes a quantity known as the Decay Energy or Q value is used to compare the energy released by a specific decay to other decays. To answer this you need to look at the binding energy per nucleon graph as follows: Fusion releases the energy of the strong force (much stronger at short distances than the EM force) when the small pieces are captured and held into one nucleus.īill Baird, Ph.D., Postdoc, College of Charleston, SC In the case of fusion, this energy is released when very light atoms are turned into. The energy per event is greater (in these examples) in fission, but the energy per nucleon (fusion = about 7 MeV/nucleon, fission = about 1 Mev/nucleon) is much greater in fusion.įission releases the energy of the electromagnetic force when positively charged parts of the nucleus fly away from one another. Nuclear fusion, like nuclear fission, can provide energy from mass. The reason why opposite processes release.
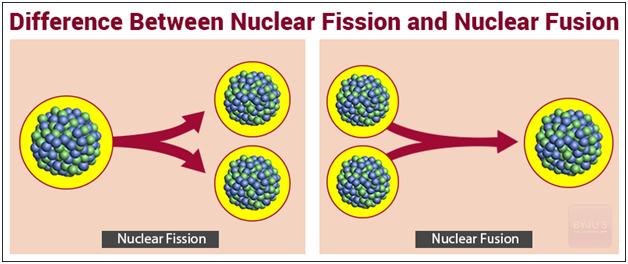
In both cases, energy is freed because the mass of the remaining nucleus is smaller than the mass of the reacting nuclei. The energy released when 4 Hydrogen nuclei (= protons) fuse (there are some decays involved as well) into a Helium nucleus is around 27 Million Electron Volts (MeV), or about 7 MeV per nucleon.įor fission of U or P, energies released are around 200 MeV or so. Fission splits a heavy element (with a high atomic mass number) into fragments while fusion joins two light elements (with a low atomic mass number), forming a heavier element. Fusion only produces more energy than it consumes in small nuclei (in stars, Hydrogen & its isotopes fusing into Helium). Why does the nuclear fusion reaction yield more energy than the nuclear fission reaction?įission only produces more energy than it consumes in large nuclei (common examples are Uranium & Plutonium, which have around 240 nucleons (nucleon = proton or neutron)).


 0 kommentar(er)
0 kommentar(er)
